Giant futuristic batteries to power 2,000 households

Renewable energy like solar and wind may help to save the planet, but a key challenge is storing the power they generate for dark or low-wind periods.
One approach for large-scale energy storage systems are redox flow batteries, which are basically large tanks with flowing electrolytes.
Researchers from several Fraunhofer Institutes are working jointly to develop these scalable fluid batteries with a goal of one day building a handball-court-sized battery installation with a capacity of 20 MWh – enough energy to provide power to roughly 2,000 households through a long winter's night or an overcast day.
They still have a long way to go. Currently, they have a working demo based on a 2-kW plant that is on display at the Hannover Messe conference this week. A 20-kW plant is scheduled to go into operation at the end of next year and the researchers hope to cross the megawatt threshold in roughly five years.
“The process already works reliably,” said Dr. Christian Dötsch in a news release. Dötsch is a business unit manager for Energy Efficiency Technologies at UMSICHT, one of the participating institutes. “The challenge lies in the upscale version, the enlargement of these plants.”
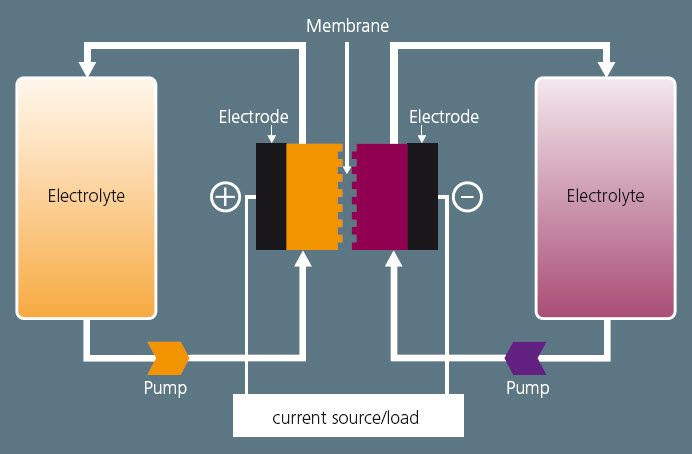
Above is a schematic of a redox-flow cell from a related article detailing the technology. The energy storage units operate in conjunction with an electrolyte tank for each of the two electrodes. The liquid electrolytes (e.g., vanadium) contain metal ions that flow through electrodes made of porous graphite fleece, separated by a membrane which is proton-permeable. During this exchange of charge a current flows over the electrodes which can be utilized.
The battery can provide unlimited capacity simply by using larger and larger storage tanks, and it can be left completely discharged for long periods with no ill effects. And because both electrolytes contain the same materials they do not contaminate the cells and the tanks if the electrolytes become mixed. They only need to differ in terms of ion charge or oxidation stage.
“This makes it possible to build very robust and durable batteries – a decisive advantage of this battery technology,” emphasizes Fraunhofer's Dr. Tom Smolinka.
On the downside, redox flow battery technology has relatively poor energy-to-volume ratio, and the system is more complex than standard storage batteries. For instance, insuring that the vanadium fluid flows smoothly through the large membranes and past the felt-like carbon electrodes in the cells themselves is a challenge, according to the researchers.
Fraunhofer researchers are convinced that the advantages of redox flow batteries will drive development, and in the next five years larger demo systems will be built and quickly followed by commercial redox-flow batteries.
Eventually, the focus will move on to the development of redox-flow batteries as a feasible technology for electric cars.