How an Android-powered artificial pancreas could revolutionise diabetes management

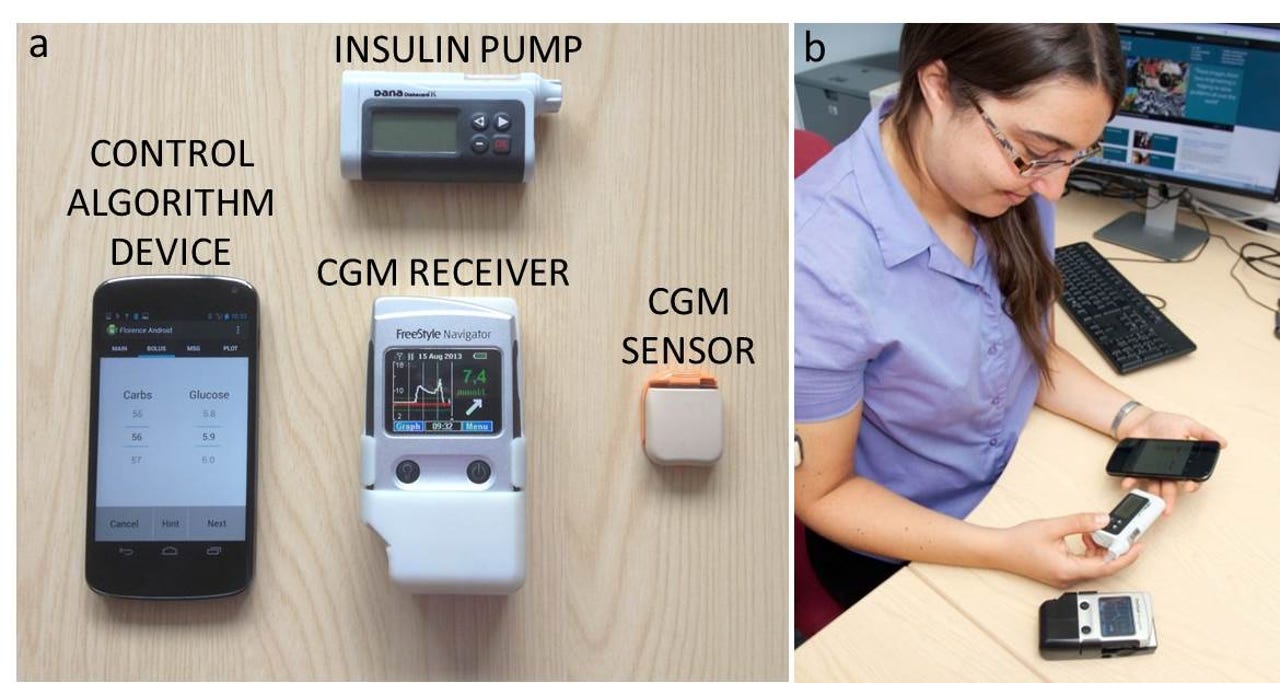
The components of the artificial pancreas, and the system in use.
You might not think an awful lot about your pancreas, because it's just getting on with its job of making sure your body has enough fuel to keep working. But if you're a diabetic, you'll be thinking about it a lot.
The pancreas is responsible for making and releasing insulin, an enzyme that helps control the level of glucose in the blood -- the substance all cells in your body use as their main source of energy. In type I diabetics, the pancreas isn't producing enough insulin. Without regular manual injections of insulin up to six times a day, diabetics' insulin levels will drop to dangerously low levels, with potentially fatal consequences.
Until now, type I diabetics have typically managed their condition by manually monitoring their blood glucose levels with simple probes, and then injecting themselves with insulin several times a day. More recently, glucose pumps have been helping to automate the process: the pumps deliver insulin when needed through a tiny tube, known as a cannula, under the skin. Diabetics still have to top themselves up with extra insulin after eating, though.
However, the way that type I diabetics keep on top of their condition could be about to change, thanks to technology. From as early as next year, medical hardware companies are expected to release devices known as "artificial pancreases": closed-loop systems that will automatically monitor the wearer's glucose level and top it up as and when needed -- courtesy of an algorithm stored on a simple Android phone.
A team researching artificial pancreas technology at the University of Cambridge has already produced a prototype of an artificial pancreas closed-loop system.
Not only does it free type I diabetics from the need to continuously track and adjust their own glucose levels, it can keep them within a much narrower range of glucose than they would be able to themselves.
While conventional glucose pump technology will stop delivering insulin when blood sugar hits a certain level or is predicted to do so, the Cambridge team's artificial pancreas prototype monitors glucose levels and suspends or increases insulin as and when needed.
"The basic idea [behind the pancreas] is to improve glucose control in people with type I diabetes, and the reason is, because it's not very good! It's not very good because our body does an amazing function of keeping glucose control very tight, by changing the amount of insulin and other hormones in the body every hour and every minute, and also day to day. That's really difficult to achieve in people with type I diabetes," Dr Roman Havorka, who leads the Cambridge University research, told ZDNet.
While diabetics might not be able to adjust their own insulin levels overnight, artificial pancreases can keep glucose levels within the desired range from hour to hour. Over the course of one night to the next, a person's insulin requirement can be anything from 30 percent of their average requirement to 300 percent of it. "This underlying variability makes conventional therapy difficult, and it's why people have episodes of low glucose," Havorka said.
While the components of the artificial pancreas have all been singly available for some time, no commercially available system has been able to link them all together.
The Cambridge prototype system, however, has. "We really tried to move quickly with available technology. There have been lot of big promises made and then reality kicks in," Havorka said. "Our idea, and the whole [artificial pancreas] movement is to reduce the existing components -- which is the continuous glucose monitor and to use the insulin pump and have a computer program which links the glucose measurement to insulin delivery," Havorka said. "It takes away the syncing process from people and does it behind the scenes."
The algorithm was built using the researchers' mathematical computer models of glucose metabolism, which are then individualised to reflect the particular diabetic patient that will wear the system, and how different doses of insulin will affect them. The optimal insulin levels are trialled in a simulation environment to test the various settings of the system.
See also
While ultimately the aim would be to make a system that's far smaller than the existing prototype, for now, artificial pancreas researchers are keen for time to market to take precedence over form factor.
In the prototype, the Cambridge University-developed system uses an Android smartphone to run the homegrown insulin-syncing algorithm. "The mobile phone is the hub for the computer program and all the communication is wireless." For trials of the prototype, the phone is locked down, but it could ultimately be made available as a common-or-garden app.
By using the phone as the lynchpin of the system, the prototype can also give diabetics or their physicians an insight into their condition through the data it gathers. "People can always see what their glucose volume is and how much insulin is being given," Havorka said.
Of course, with any technology, there's a risk of failure -- the phone needing a reboot at a critical time, a sensor goes dark, a cannula gets blocked. If something goes wrong the system will set off an alarm to let the wearer know their glucose level has dropped too low. Researchers are currently working out the best way to use those alarms to alert patients of problems without causing "alarm fatigue", where users grow too accustomed to the warnings and begin to ignore them.
Before the artificial pancreas systems can be released, they'll need to undergo clinical studies to prove they're safe for use. The Cambridge group has already had diabetics use the system in their own homes without medical supervision, in groups including children, adults, and pregnant women.
"These are really free-living studies where people drive, do exercise, go on holiday, go to school. These studies are ongoing now, and we're planning longer studies" of up to one and three years, Havorka said.
The hope is that the technology won't just deliver physiological benefits either: it'll make the lives of diabetics and their families that bit easier too by taking the hassle out of managing the condition -- particularly important for young people and teenagers who can find adherence to treatment difficult. "The overall wellbeing of people improves, the stress in the family is reduced, and people should be living more normal lives," Havorka said.
The first closed-loop system is expected to be approved by the FDA next year, with commercials units released not too long after that.
"The FDA is really supportive of that technology reaching people with type I diabetes. The FDA approach is accelerated, they are saying people with type I diabetes have everyday risks of untoward occurrences and adverse glucose values, and the whole community is willing to accept these systems because traditionally if any treatment comes out, it needs to be shown to be bulletproof They're saying the community is willing to accept things that aren't bulletproof and we won't stand in the way. It's amazing."
Read more health tech stories
- Bioprinting bones and muscles: The inkjet cell printers shaping the future of transplants
- Rebuilding the brain: Using AI, electrodes, and machine learning to bridge gaps in the human nervous system
- Seven ways AR and VR are changing the healthcare world (TechRepublic)
- No digital Swiss Army knife on horizon for health wearables market (CNET)