Research gives clues for self-cleaning materials, water-striding robots

Self-cleaning walls, counter tops, fabrics, and even micro-robots that can walk on water may all be within reach thanks to the work of scientists at the University of Nebraska-Lincoln and at Japan's RIKEN institute.
If you ever looked in awe as insects like water striders are able to walk effortlessly on water, it's because of a property called super hydrophobia. It's been a subject studied by scientists and engineers for decades. These insects have water-resistance legs that are covered by large numbers of oriented tiny hairs (microsetae) with fine nanogrooves. With help from a wax coating on their legs, it's this physical structure that allows them to glide over the water without sinking in.
"Their legs are super hydrophobic and each leg can hold about 15 times their weight." says Xiao Cheng Zeng, Ameritas university professor of chemistry at UNL.
Now, researches are publishing some important clues in how to develop similar surfaces with super hydrophobic materials. These findings will appear in a paper to be published in the May 4-8 online edition of the Proceedings of the National Academy of Science.
Before we jump into the findings, it is first important to understand just how super hydrophobics occurs in nature.
A news item from UNL explains: "Organisms like caterpillars, water striders and the lotus achieve super hydrophobia through a two-level structure -- a hydrophobic waxy surface made super hydrophobic by the addition microscopic hair-like structures that may be covered by even smaller hairs, greatly increasing the surface area of the organism and making it impossible for water droplets to stick."
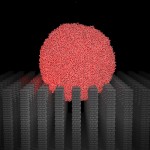
To create a similar surface, Zeng and his team turned to the super-fast supercomputer at RIKEN rather than face a slow and tedious attempt to find the right nanostructure in a lab. They designed a computer simulation to perform tens of thousands of experiments that studied how surfaces behaved under many different conditions. The UNL article writes: "Zeng and his colleagues used the RIKEN computer to "rain" virtual water droplets of different sizes and at different speeds on surfaces that had pillars of various heights and widths, and with different amounts of space between the pillars."
With the speed of the RIKEN computer, they performed countless repetitions with highly-controlled variables until they found what they were looking for:
What they learned is that there is a critical pillar height, depending on the particular structure of the pillars and their chemical properties, beyond which water droplets cannot penetrate. If the droplet can penetrate the pillar structure and reach the waxy surface, it is in the merely hydrophobic Wenzel state (named for Robert Wenzel, who found the phenomenon in nature in 1936). If it the droplet cannot penetrate the pillars to touch the surface, the structure is in the super hydrophobic Cassie state (named for A.B.D. Cassie, who discovered it in 1942), and the droplet rolls away.
According to Zeng, "In the Cassie state, the water droplet stays on top and it can carry dirt away. In the Wenzel state, it's sort of stuck on the surface and lacks self-cleaning functionality. When you build a nanomachine -- a nanorobot -- in the future, you will want to build it so it can self-clean."
It will be interesting to see what applications may come out of this research, but as for nanorobots, it's probably a good thing someone is thinking about how to keep them clean.
Along with Zeng, Takahiro Koishi of the University of Fukui and RIKEN, Kenji Yasuoka of Keio University, and Shigenori Fujikawa and Toshikazu Ebisuzaki of RIKEN, participated in the study with help from the Nebraska Research Initiative, the Department of Energy and the National Science Foundation.