New long-lasting 'battery' can charge in seconds, doesn't degrade

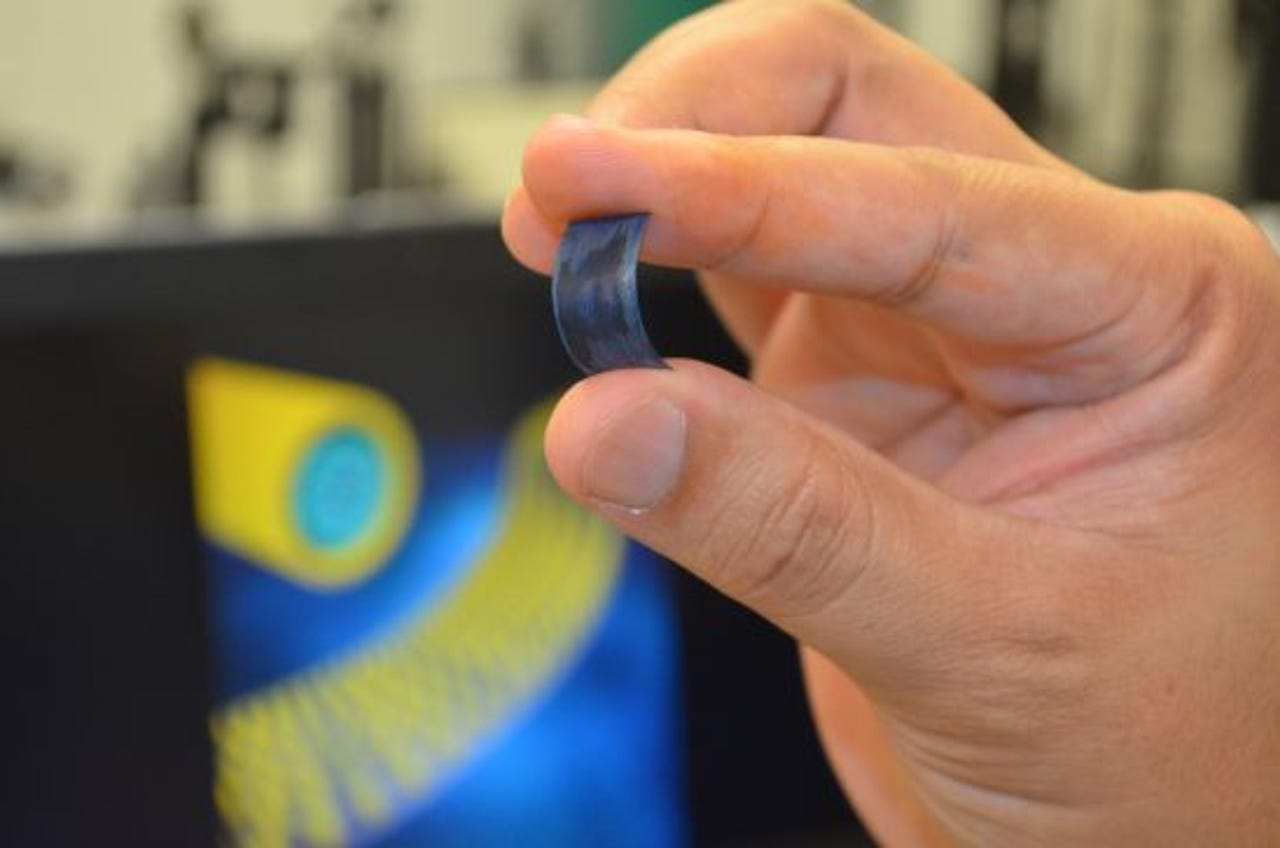
This tiny, flexible supercapacitor can charge in seconds.
Scientists have developed a method for creating small, flexible supercapacitors that could mean blisteringly fast charging times and more reliable batteries.
While lithium-ion batteries can break after about 1,500 charges, this supercapacitor can be recharged 30,000 times before degrading, according to scientists at the University of Central Florida. Better yet, the supercapacitor can charge in a blink of an eye and wouldn't need topping up for a week.
Supercapacitors use static electricity to store energy, as opposed to batteries which use an electrochemical reaction.
"If they were to replace the batteries with these supercapacitors, you could charge your mobile phone in a few seconds and you wouldn't need to charge it again for over a week," said Nitin Choudhary, a postdoctoral associate and one of the main authors of a new paper detailing the process.
The supercapacitors they've created are also flexible, which could help address one of the main pitfalls of devices such as the Apple Watch.
Historically, one of the main disadvantages of supercapacitors is that they hold far less energy than a similarly-sized lithium-ion battery. So researchers have been exploring the use of nanomaterials, such as graphene, to improve capacity.
As noted by Engadget, supercapacitors store electricity statically on the surface of a material and require two-dimensional material sheets with enough surface area to hold lots of electrons.
Yeonwoong 'Eric' Jung, an assistant professor at UCF and nano-materials researcher, said the chief problem with existing approaches has been in integrating these two-dimensional materials into existing systems.
"That's been a bottleneck in the field. We developed a simple chemical synthesis approach, so we can very nicely integrate the existing materials with the two-dimensional materials," Jung said.
Their supercapacitors are packed with millions of nanometer-thick wires wrapped in two-dimensional materials.
"A highly-conductive core facilitates fast electron transfer for fast charging and discharging. And uniformly coated shells of two-dimensional materials yield high energy and power densities," the university explains.
Jung is in the process of patenting the method. However, he warned it could be some time before this technology is seen in electronic gadgets and vehicles.
"It's not ready for commercialization," Jung said. "But this is a proof-of-concept demonstration, and our studies show there are very high impacts for many technologies."
It's unclear whether the supercapacitors would be suitable for replacing lithium-ion outright or complementing them, since they're ideal for providing energy when vehicles or devices need sudden bursts of power and speed.
However, Choudhary said the technology is already outperforming lithium-ion on some measures in small electronic devices.
"For small electronic devices, our materials are surpassing the conventional ones worldwide in terms of energy density, power density and cyclic stability," he said.