Bionic pancreas keeps blood sugar levels just right

Type 1 diabetes is an autoimmune disease that destroys the pancreatic cells that produce insulin, which is necessary for the body to convert carbs to energy. As a result, sugars build up in the blood, which can damage tissue and organs over time.
The bionic pancreas is able to control blood sugar using doses of insulin to lower blood glucose, as well as doses of glucagon, a hormone that raises blood glucose. The two work together to prevent levels from reaching dangerous levels.
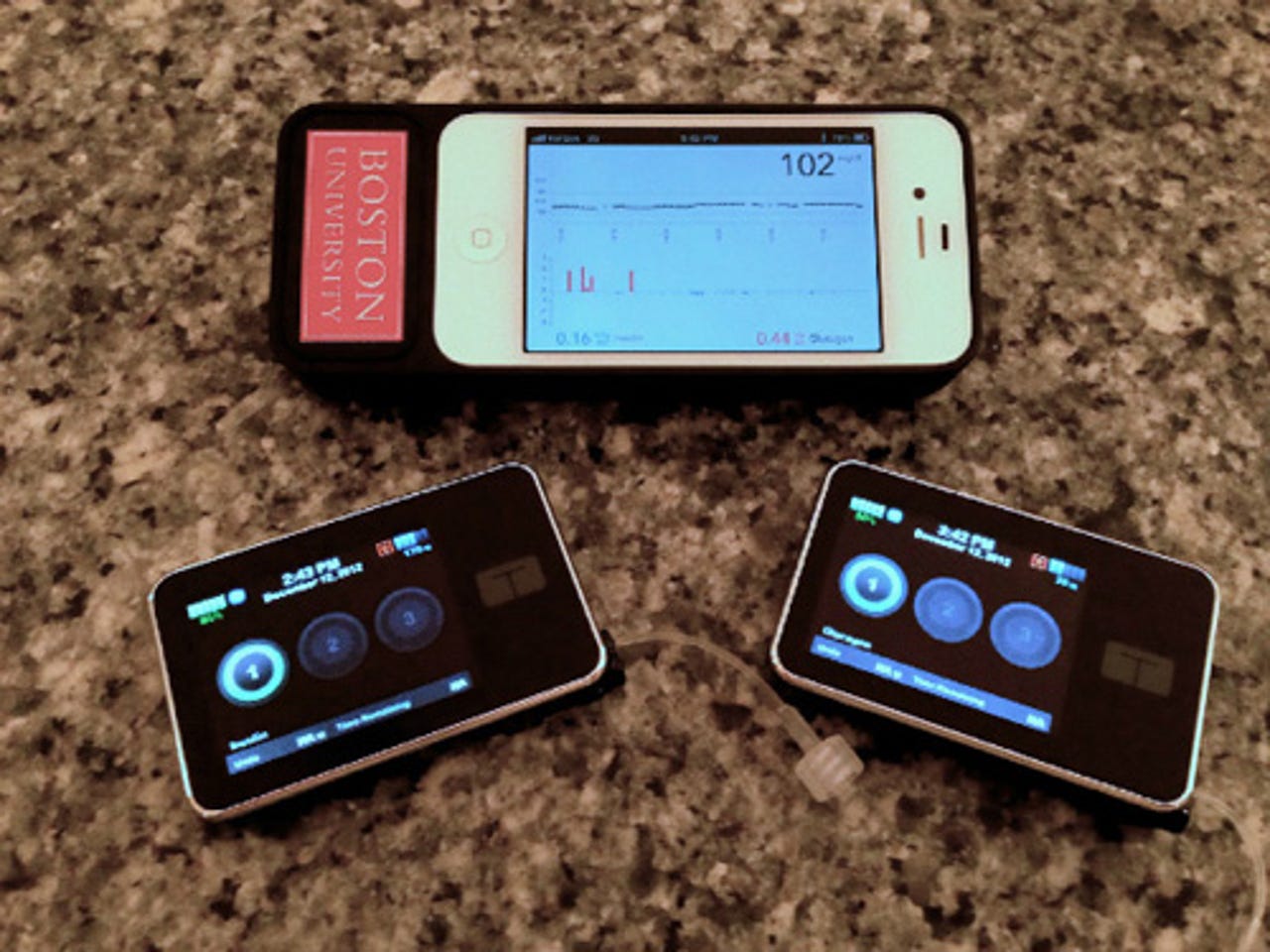
Developed by researchers from Boston University and Massachusetts General Hospital, the new system consists of two pumps (pictured above) that are attached to the abdomen, a computer algorithm on an iPhone 4S, and a continuous glucose monitor with a wire just under the patient's skin.
Every 5 minutes, the phone receives a blood sugar reading from the glucose monitor. Then the phone app calculates and automatically administers the dose of insulin or glucagon by wirelessly communicating with the pumps. The system continually adapts its decisions about insulin and glucagon dosing.
The latest trials, conducted in 2013, enrolled 20 adults and 32 adolescents ages 12 to 20. The adults lived at home and managed their own care, while the teens were monitored while at camp. The mobile system performed better than conventional pumps, which required a finger prick and manual calculations of dosage.
"The most practical difference would be not having to think about diabetes 24/7, not having to constantly make decisions about things that those of us without type 1 never have to think about," study coauthor Edward Damiano of Boston University says in a news release. "Another real problem that would be relieved is the fear -- fear of going to bed at night and not knowing if your blood sugar level will drop dangerously low while you sleep."
Damiano, whose 15-year-old son was diagnosed with type 1 diabetes when he was 11 months old, was first motivated by the fear that the child's blood sugar would dive while he was sleeping, called dead-in-bed syndrome. Two follow-up trials are in the works. Damiano's goal is to have the device approved by the U.S. Food and Drug Administration by the time his son goes to college in 2017.
The work was published in the New England Journal of Medicine last week and presented at the American Diabetes Association Scientific Sessions in San Francisco.
[MGH, BU via New Scientist]
Image: Boston University Department of Biomedical Engineering
This post was originally published on Smartplanet.com